Rome II criteria defines constipation as less than 3 defecations per week associated with straining, lumpy or hard stools and sensation of incomplete evacuation. It has been observed that storage, transport and evacuation mechanism of colon is disturbed in constipation which hampers the normal bowel movement leading to passage of lumpy or hard stools and sensation of incomplete evacuation. Constipation increases with age and is more common in women than men in all age groups. Constipation is also a frequent but short lived problem in infants. Risk factors for constipation includes old age, inadequate dietary fiber, lack of exercise, pregnancy, abuse of laxatives, suppression of defecatory urges arising at inopportune moments, specific diseases such as piles and fistula irritable bowel syndrome etc. Softovac® is a herbal bowel regulator developed by Lupin limited to treat constipation. In this study, safety and efficacy of Softovac® was evaluated in patients with constipation.

Study Type: Open Label, non-comparative clinical trial
Sample Size: 30 patients of both sex and aged between 18-60 years
Intervention: Softovac® powder. It contains Isaphgol (Plantago ovata), Sonamukhi (Senna), Harad (Terminalia chebula), Amaltas (Cassia Fistula), Mulethi (Licorice), Gulab Dal (Rose Petals), Saunf (Fennel Seeds), Saunf Taila (Fennel Oil), and Sharkara (Sugar).
Inclusion Criteria: Patients having less than three bowel movements in a week and two or more associated symptoms such as straining, sense of incomplete evacuation, hard and lumpy stool. Exclusion criteria: Patients with history of any surgery of gastrointestinal tract, patients suffering from metabolic, cardiac, respiratory, neurological and psychiatric illness, pregnant & lactating women were excluded.
Treatment:: Softovac® powder 5-10 g, every night for 14 days (follow-up at day 7 & day 14)
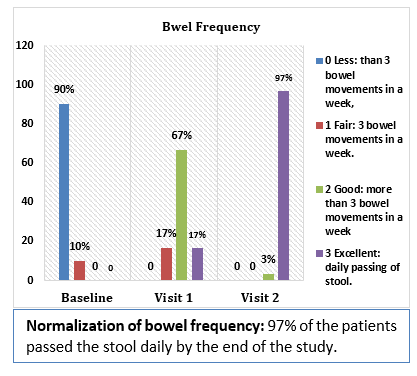
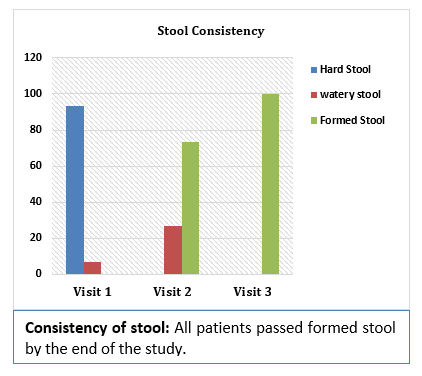
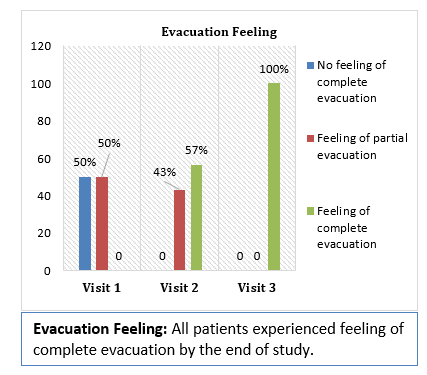
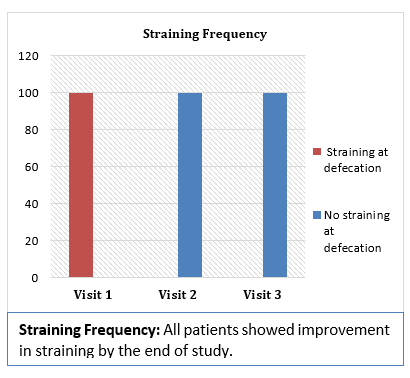
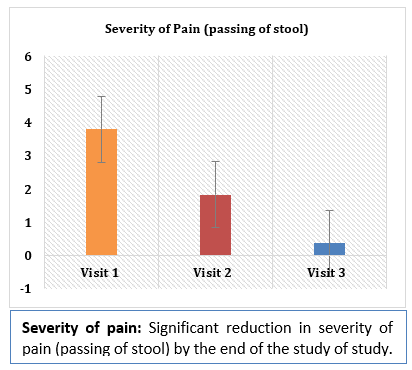
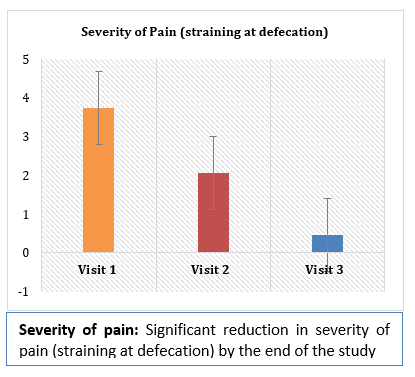
Results and Conclusion: Consumption of Softovac® significantly improved constipation symptoms
including bowl frequency, stool consistency, feeling of evacuation and straining frequency. It also
significantly reduced severity of pain during defecation. No serious adverse events (SAE) or side
effects were reported during the treatment with Softovac®. During the study the need for intake of
other laxatives didn't arise in any patients.
Softovac® is a complete herbal formulation with bulk forming, mild stimulant, laxative, demulcent
and lubricating properties. Softovac® regulates normal peristalsis and possesses carminative anti-
flatulent and carminative properties. Softovac® in short course (15 days) effective and safe with
excellent patient compliance.
References: Malgi SV, Gaikwad SS. Clinical study of Softovac- A Herbal Bowel Regulator for the Treatment of Constipation. Indian Medical Gazette. August 2016: 290-300.
Results :